Just like with boiling points the presence of polar and hydrogen-bonding groups on organic compounds generally leads to higher melting points. Melting point of Hydrogen is -2591C.
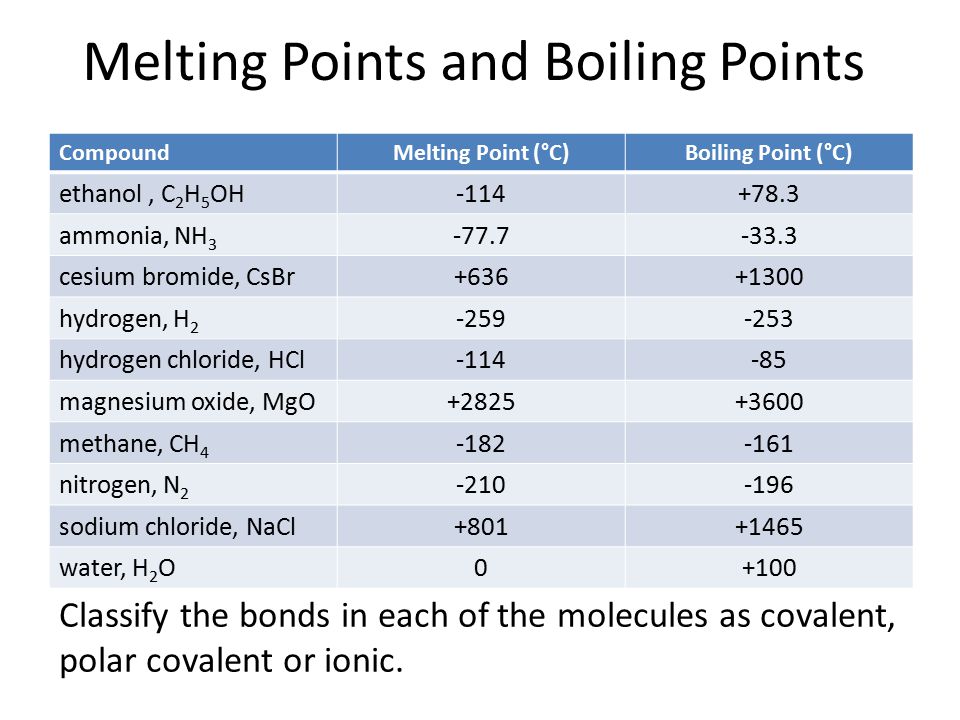 Melting Point And Boiling Points Of Ionic And Covalent Compounds Ppt Video Online Download
Melting Point And Boiling Points Of Ionic And Covalent Compounds Ppt Video Online Download
Compare boiling points of ethanol and ethyl chloride compounds.
Hydrogen melting and boiling point. Hexagonal Density 293 K. Ice is less dense than water Generally the solid form of a material will have a greater density than the liquid. A molecule that can form more hydrogen bonds with more neighboring molecules will have more extensive hydrogen bonds hence more energy is required to break more hydrogen bonds per molecule and the meltingboiling point is higher.
Note that these points are associated with the standard atmospheric pressure. Again due to the presence of hydrogen bonds in Hydrogen Fluoride HF molecule its melting and boiling points are higher. However at very low temperature andor high pressures the gas becomes a liquid or a solid.
The major reason for this abnormal behavior is the strong attractions afforded by the hydrogen bonds. Freezing and Melting Points for common Liquids - Common fluids and their freezing and melting points. Ethyl chlorides boiling point is 123 0 C.
The other molecules are slightly polar and show the increase in boiling point with molecular weight which is normal. Thermal conductivity of Hydrogen is 01805 WmK. When number of hydrogen bonds and strength of hydrogen bonds increases melting and boiling points increases.
The melting point of para -hydrogen is 010 lower than that of a 31 mixture of ortho -hydrogen and para -hydrogen. Note that these points are associated with the standard atmospheric pressure. Fluorine has a higher electronegativity than the other halogens which means for fluorine it undergoes hydrogen bonding which gives it a boiling point of about 19 degrees Celcius.
Hydrogen Thermal Conductivity. The curve between the critical point and the triple point shows the hydrogen boiling point with changes in pressure. Making matter more stable Hydrogen bonds make matter more stable.
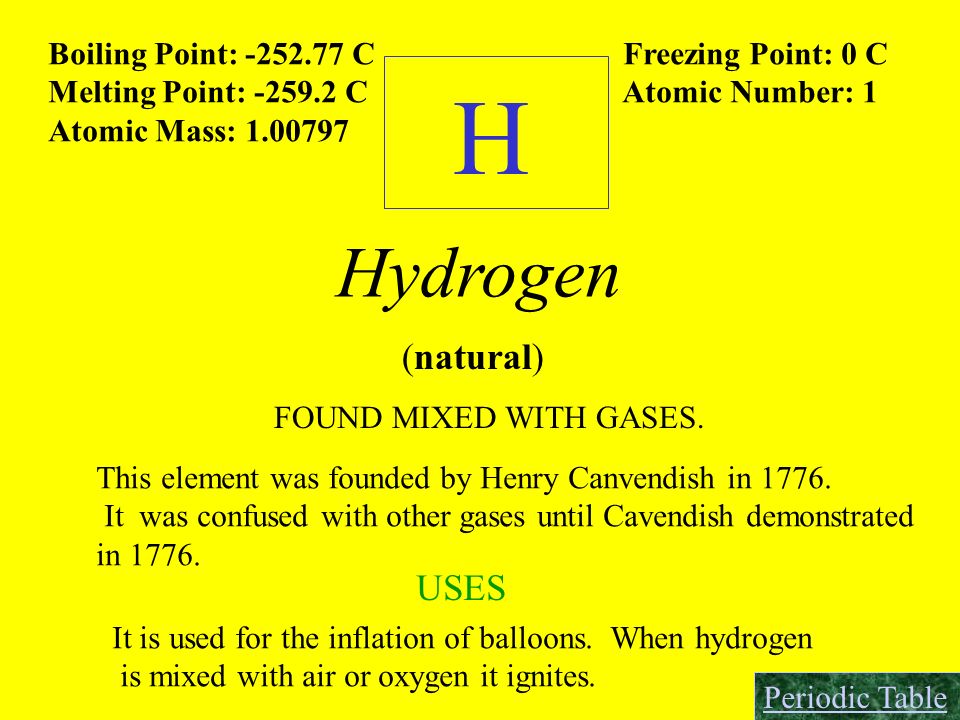
Hydrogen Melting Point Melting point of Hydrogen is -2591C. The size of a molecule influences its melting point as well as its boiling point again due to increased van der Waals interactions between molecules. Both organic compounds have two carbon atoms.
Boiling point of Hydrogen is -2529C. Whenever you are trying to changes phases it takes energy to overcome those strong inter molecular forces. Extensiveness is related to the maximum number of hydrogen bonds a molecule can form with its neighbours.
High melting and boiling points of the compounds containing hydrogen bonds is due to the fact that some extra energy is needed to break these bonds during the process of melting and boiling. Hydrogen chloride has a simple molecular structure. This requires very little energy and thus the melting point is low.
In this post we will talk about the melting and boiling points of organic compounds and their correlation with intermolecular forces such as dipole-dipole London dispersion also known as Van der Waals interactions and hydrogen bondingWe discussed these infractions in the previous post and today the focus will be more from the perspective of physical properties. Hydrogen is a gas at standard conditions. 100794 amu Melting Point-25914 C 14009985 K -43445203 F Boiling Point-25287 C 20280005 K -423166 F Number of ProtonsElectrons.
1 Number of Neutrons. Hydrocarbons - physical data - Molweight melting and boiling point density flash point and autoignition temperature as well as number of carbon and hydrogen atoms in each molecule are given for 200 different hydrocarbons. A stronger Hbond exists in HF than in water yet water boils at higher temperature than HF.
These forces are in addition to the VDW forces already present. Hydrogen Melting Point and Boiling Point Melting point of Hydrogen is -2591C. In turn the melting point is raised to compensate.
Boiling point of Hydrogen is -2529C. The hydrogen phase diagram shows the phase behavior with changes in temperature and pressure. Ethanols boiling point is 7837 0 C.
The atoms within the molecule are held together by strong covalent bonds. Thermal Properties of Hydrogen Hydrogen Melting Point and Boiling Point. Instead water boils at 100 C which is very abnormal.
Relatively high melting and boiling points There are strong hydrogen bonds between the molecules of water. However for simple covalent molecules to melt only the weak intermolecular forces need to be overcome not the strong covalent bonds. This means that extra energy is required to overcome these hydrogen bonds to melt or boil the water.
008988 gcm 3 Color. The two forms of hydrogen have slightly different physical properties.
Toggle Navigation Who We Are. Thus the boiling point is dependent on the pressure.
 Solved 1 Suppose A Student Measuring The Boiling Tempera Chegg Com
Solved 1 Suppose A Student Measuring The Boiling Tempera Chegg Com
Marmalade cooked to the setting point 222F 1055oC is chewy and very thick.

What is the boiling point of orange juice. The freezing point of orange. Ethyl butyrate also known as ethyl butanoate or butyric ether is an ester with the chemical formula CH 3 CH 2 CH 2 COOCH 2 CH 3It is soluble in propylene glycol paraffin oil and keroseneIt has a fruity odor similar to pineapple and is a key ingredient used as a flavor enhancer in processed orange juices. That freezing point would correspond to an initial boiling point of about 10032C.
Pasteurise the juice by immersing batches of the. It also occurs naturally in many fruits albeit at lower concentrations. Add boiling water sugar and lemon juice to the pulp so that the mixture contains 12 total soluble solids as measured by a refractometer and has a pH of 35 -38.
It reaches its boiling point at a higher temperature than water due to its sugar content. Theres a lot of sugar in it so that lowers the melting point. As solute molecules are added to water the boiling point increases.
For water it was 100 degree Celsius for sugar solution it was 104 degree Celsius and with the highest boiling point salt solution had 106 degree Celsius More heat energy was needed to break the ionic bond in salt solution than the covalent bond in sugar solution so the boiling point of salt solution was higher. Apple juice begins to boil when it reaches a temperature of 160 degrees Celsius or 320 degrees Fahrenheit. I bet youll have to heat it to very roughly 105C to get rid of all the liquid.
At sea level the standard atmospheric pressure is 101325 mbar and the boiling point of water is 100 C 212 F. The orange juice concentrate has at least 35 solids including pulp non-volatile compounds pectin and volatile compounds. Data on the rise in boiling points of fruit juices at different concentrations were presented by Ilagantileke et al.
Take a trip to the mile-high city of Denver and the pressure drops to 805 mbar and water boils at 937 C 2007 F. At 222F the peel is a nice chewy. You could use pH paper to measure and compare the pH of each liquid.
Even though the sugar is not really part of the ice its sitting in little pockets throughout the ice helping melt it. Boiling points may be published with respect to the NIST USA standard pressure of 101325 kPa or 1 atm or the IUPAC standard pressure of 100000 kPa. This orange juice concentrate has at least 65 of the aroma and flavor volatile compounds of the natural juice.
The caramel undertone is coming through and theres a bit of a bitter orange flavour that lingers. Likewise orange juice having spores of Bacillus subtilis has been studied with the help of alternating current electric field Uemura Isobe 2003. That raises its boiling point.
This is because orange juice is predominantly made of water most brands are around 88 waterThe boiling point of orange juice When you heat orange juice to 100 degrees Celsius the water content in the juice starts to evaporate leaving behind all the concentrate and other additives that make your orange juice taste like orange juiceObviously the ratio of water to pulp and concentrate might have an effect on how fast your orange juice boils. 5 for Thai tangerine juices by Crapiste and Lozano 6 and Moresi and Spinosi7 for apple juice by Moresi and Spinosi3 for orange juice and by Varshney and Barhate8 for pineapple mango and lemon juices. Although the chemical formula for THC C 21 H 30 O 2 describes multiple isomers the term THC usually refers to the Delta-9-THC isomer with chemical name -trans-D⁹-tetrahydrocannabinol.
For one method of measuring conductivity see the Science Buddies project idea Electrolyte Challenge. Again as the water boils away that leaves more concentrated sugars and salts in the remaining liquid. Like most pharmacologically active secondary metabolites.
A natural orange juice concentrate prepared from natural orange compounds is disclosed. Tetrahydrocannabinol THC is one of at least 113 cannabinoids identified in cannabisTHC is the principal psychoactive constituent of cannabis. However on the basis of the above con- previous thermodynamic considerations the experimental re- siderations it can be estimated that in an apple juice pH sults were in disagreement with published data for orange juice 35 heated from 40 to 100C for approximately 1 hr which Moresi and Spinosi 1980 and pineapple mango and lemon represents the time elapsed for determining the boiling point juice Varshney and Barhate 1978.
Lima Heskitt Burianek Nokes and Sastry 1999 determined vitamin C reduction kinetics using ohmic heat treatment to heat orange juice with an electric field of 182 Vcm for 30 min at 90C. Orange JUICE boils at 100 degrees celcius 212 Farenheit or 373 Kelvin because orange JUICE is 88 water Hope that helped Whats the freezing point of orange juice. The boiling point corresponds to the temperature at which the vapor pressure of the liquid equals the surrounding environmental pressure.
This is the upper limit in my opinion as beyond this point the peel gets really really chewy. Pure water boils at 100C at normal atmospheric pressure. Extract the pulp using a hand-driven or electrical juice extractor.
As you say it makes sense that the cranberry juice melted first. The same applies to the milk which I guess doesnt have quite as many molecules dissolved in it. Fill the juice into clean sterilised bottles and seal with caps.
Handheld wireless solution for test ing blood gas electrolytes and metabolics. Check out our list of supported devices or make a request.
 Statsensor Creatinine Point Of Care Monitoring System From Nova Biomedical Labcompare Com
Statsensor Creatinine Point Of Care Monitoring System From Nova Biomedical Labcompare Com
Visbys testing device has palm-sized dimensions and eliminates the need for an additional instrument or reader so that it can provide fast accurate results at the point of care requiring only.

Point of care testing device. Point of care testing POCT is defined as a medical diagnostic testing that allows medical staff and physicians to accurately achieve real-time lab-quality diagnostic results within minutes. These range from simple battery operated strip devices to more complex bench-top devices. Point of care diagnostics can integrate biochemical testing alongside physiologic measurements.
There is an ever-increasing array of POCT available most of which are beyond the remit of this article which will concentrate upon those commonly used and relevant to the management of perioperative coagulation. In general the term can encompass any patient medical test that is given ad hoc and provides quick results. This study validated the performance and utility of a handheld point-of-care POC lactate device in comparison with the lactate and pH values obtained by the ABL 800 blood gas analyzer.
Many devices are now available to carry out testing at the actual point of care thus avoiding delays in diagnosis. A near patient in vitro diagnostic device IVDD is used for. Point-of-care testing POCT may be defined as the rapid specific testing of bodily fluids at the bedside.
We connect with the most popular point-of-care testing devices both CLIA waived and nonwaived. Through the use of portable blood analysers testing at the point of care streamlines the diagnostic process and helps ensure patients receive the most effective and efficient care when and where it is needed. Point-of-care testing in a health care setting for example doctors office pharmacy at the bedside or self-testing for example used by individuals at home Typically these rapid test devices are simple to use and provide visual or simple results within a short time.
Requires no instrumentation and provides results in 15 minutes making it a valuable tool for mass testing in decentralized settings. On the device in the electronic healthcare record on a patientward monitor to the clinician on the move and directly to the patient. POC tests can also extend testing to people residing in communities who cannot readily access care.
The research report published by Future Market Insights on the Point Of Care Blood Testing Devices Market provides a detailed overview of the demands and consumptions of various productsservices associated with the growth dynamics of the market during the forecast period 2017-2027. The clinical performance and influences on accuracy and decision-making criteria were assessed with freshly taken fetal blood scalp samples n57 and. Most POC testing devices are handheld electronics or molecular collection tools.
Point-of-care testing is defined as medical diagnostic testing at or near the point of carethat is at the time and place of patient care. An advanced easy-to-use blood analyzer that provides healthcare professionals with access to lab-quality results in minutes for point-of-care testing. The in-depth market estimation of various opportunities in the.
FebriDx can be used to help triage patients at the point of care to reduce uncertainty and avoid unnecessary antibiotics. POCT Testing Point of care POC diagnostic devices are used to obtain diagnostic results while with the patient or close to the patient. Weve worked in partnership with CDP since April last year to undertake accelerated pilot manufacture of our Q-POC device which is a portable DNARNA analyser offering rapid sample-to-answer molecular diagnostic testing at the point of care.
Point of Care Testing Market size exceeded USD 242 billion in 2019 and is estimated to grow at over 71 CAGR between 2020 and 2026. Point-of-care tests such as some rapid tests for diagnosing an infectious disease provide results within minutes of the test being administered allowing for rapid decisions about patient care. Used in doctors offices hospitals and in patients homes POC diagnostic devices give quick feedback on many sorts of medical tests.
POC testing is also known as bedside testing near-patient testing remote testing mobile testing and rapid diagnostics. This contrasts with the historical pattern in which testing was wholly or mostly confined to the medical laboratory which entailed sending off specimens away from the point of care and then waiting hours or days to learn the results during which time care must continue without the desired information. Colin Toombs VP Research Development at QuantuMDx said.
Colin Toombs VP Research Development at QuantuMDx said. Roche Point of Care delivers those solutions meeting the clinical need for quick and accurate test results delivered where needed when needed. The first and only rapid all-in-one point-of-care test device that can identify a clinically significant acute respiratory infection ARI and differentiate viral from bacterial causes.
Weve worked in partnership with CDP since April last year to undertake accelerated pilot manufacture of our Q-POC device which is a portable DNARNA analyser offering rapid sample-to-answer molecular diagnostic testing at the point of careThe QuantuMDx and CDP teams have worked in close partnership to optimise our. Panbio COVID-19 Ag Rapid Test Device Abbott Point of Care Testing. Results in 10 minutes increases confidence in whether or.
Common examples of POC test tools include blood glucose monitors thermometers home pregnancy tests and rapid strep tests.